Effective Date: April 21, 2025
Last Update: June 26, 2025
These Terms and Conditions (“Terms”) govern your use of the services provided by Livy Health GmbH (”Livy”, “Aniva,” “we,” “us,” or “our”), a company registered in Berlin, Germany, with its registered office at Retzbacher Weg 44, c/o Noah Petermann, 13189 Berlin, Germany (Handelsregister HRB 272567, Amtsgericht Berlin-Charlottenburg). By creating an account or using Aniva’s website, mobile application, and related offerings (collectively, the “Services”), you agree to be legally bound by these Terms. If you do not agree, you must not use the Services.
These Terms, together with our Privacy Policy and any Informed-Consent documents you sign for specific tests, form the entire agreement between you and Aniva and supersede all prior agreements, discussions, or representations.
Aniva operates a digital wellness platform that allows you to
We provide these Services through accredited Partner Providers (laboratories, phlebotomy networks, logistics firms, tele-wellness coaches, and supplement formulators).
Aniva is not a medical facility or healthcare provider. We neither diagnose nor treat disease. All clinical procedures (e.g. blood draws) are performed by independent Partner Providers who are solely responsible for meeting healthcare-professional standards.
Using the Services does not create a doctor-patient relationship with Aniva. Information provided is general wellness and educational content. Always consult a licensed physician before acting on any information.
We target residents of the European Union, currently focusing on Germany and Finland. If you access the Services from elsewhere, you are responsible for local legal compliance.
You must be 18 years or older (or the age of majority in your jurisdiction) and legally able to contract. Minors may participate only through a parent/guardian account (see 3.5).
You agree to provide accurate, current, and complete registration data and to keep it updated. Each person may hold one account.
You are responsible for all activity under your credentials. Notify hello@aniva.health immediately of any unauthorised use. We may require multi-factor authentication.
You agree not to:
Parents/guardians may create accounts for minors (< 18) by:
When the child turns 18, we request re-consent; failure to respond may lead to account suspension and sample destruction.
Each test requires electronic signature of a Biospecimen Informed Consent that complies with the German Genetic Diagnostics Act (GenDG), GDPR Art. 9, and ISO 15189.
By submitting a sample, you warrant that it is yours (or that of your authorised dependent), you are not acting for an insurer/employer/law-enforcement entity, and you will not use results discriminatorily.
Current DNA panels exclude disease-risk markers. Future predictive tests will require a physician order and genetic counselling per GenDG §§ 7–11.
Risks include needle discomfort, lab errors, data breaches, psychological impact, and incidental findings.
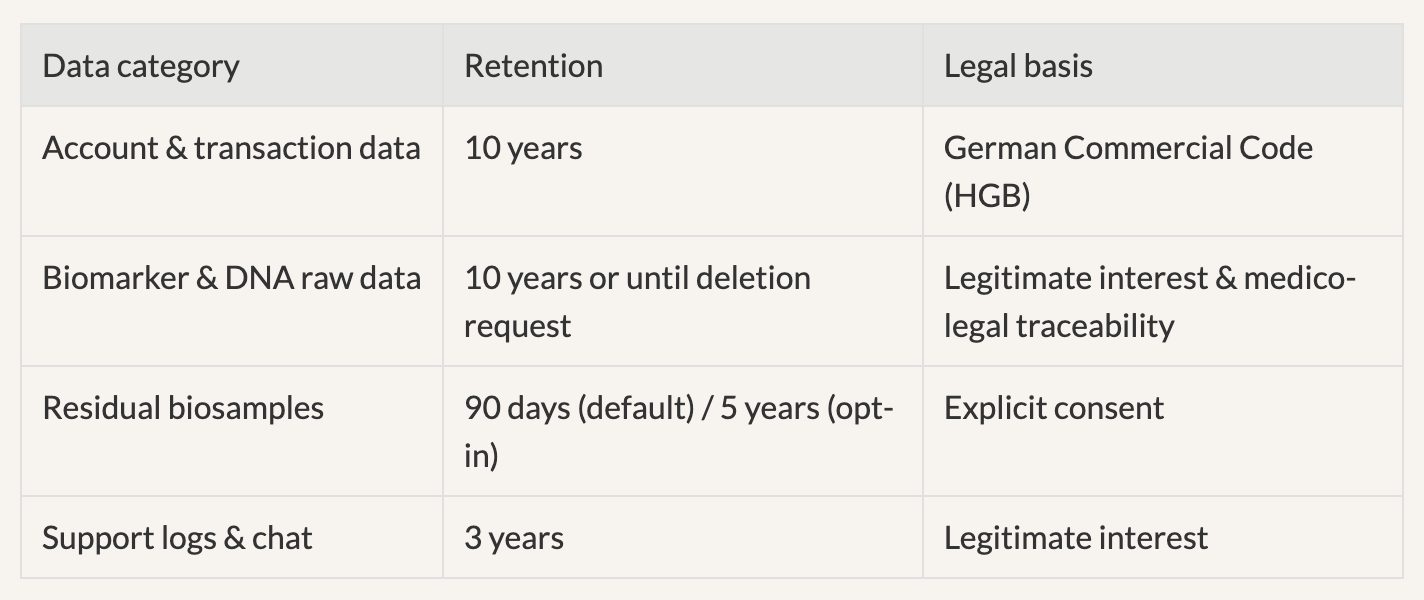
Email privacy@aniva.health to withdraw. Processing already completed cannot be reversed; residual samples are destroyed within 30 days.
Controller: Livy Health GmbH | DPO: privacy@aniva.health
Contract (Art 6 (1)(b)), explicit consent for health/genetic data (Art 9 (2)(a)), legitimate interest (Art 6 (1)(f)).
All Partner Providers sign GDPR-compliant DPAs covering purpose limitation, confidentiality, sub-processor controls, and deletion.
AES-256 encryption at rest, TLS 1.3 in transit, strict RBAC, annual ISO 27001 audits, and regular penetration testing.
Safeguarded under EU Standard Contractual Clauses 2021/914 plus encryption and pseudonymisation.
Access, rectification, erasure, restriction, objection, portability, and DPA-level complaints. Request via in-app portal or email.
All information is educational and not medical advice. Outcomes are not guaranteed and depend on individual factors.
Laboratories: ISO 15189 or DIN EN ISO 17025.
Phlebotomy partners: ≥ € 5 million professional-liability cover.
Report sample loss, lab error, or injury to quality@aniva.health within 48 h.
Partner Providers are independent contractors; Aniva is not liable for their professional acts except for negligent selection (culpa in eligendo).